Click Chemistry Peptides
Click chemistry is suitable for production of synthetic peptides.
Click peptides refer to synthesized peptides by click chemistry. This technology has characteristics of high yielding, selectivity, carbon-hetero bond, wide scope and simple performance. Click reactions in peptide chemistry are critical for peptide synthesis and chemoselective modification. The broad application of click reactions includes ligation, cyclization, bio-conjugation, and radiolabeling.
The most regular encountered functional groups for click chemistry are azides and alkynes. The azide-alkyne cylcoaddition is the most popular click reaction. Click chemistry has wide applications in chemical synthesis, materials sciences, chemical biology, and drug development.
Peptide click chemistry is also used in biological molecule labeling, covalent link to target site with high selectivity, biocunjugation in proteomics research. As the development of peptide-based drugs in research and clinical area, click peptides provide lower manufacturing costs and higher stability.
Click Chemistry
Click chemistry applies the modular strategy to generate substances quickly and reliably by joining small units together. The click reaction is highly efficient, stereospecific, and wide in scope. Click reactions are simple to perform by inexpensive reagents, and conducted in benign solvents. Click chemistry is a popular technique for custom peptide synthesis, it generates complex molecules rapidly and reliably by small units’ combination. This advanced technique has tremendous potential to modify peptides and proteins. Especially in group attaching of ligands, lipophilic or lipophobic groups, or hydrophilic and hydrophobic linkers.
Triazoles from click reactions can mimic peptides and disulgide bonds, build secondary structure, link functional groups and bio-conjugation. Click chemistry discoveries the new synthetic approaches for peptides, this technology converts peptides into minimized structure and more stable forms.
CuAAC Click Chemistry
The CuAAC (copper-catalyzed azide-alkyne cycloaddition) click reaction from a triazole link by clicking an alkyne-modified peptide with an azide-modified molecule. This reaction is high efficient, stereospecific, simple performance, and widely application scope. Click reactions have high energy contents, these reactions are irreversible and involve carbon-heteroatom bonding process. Furthermore, azide moieties are easy to introduce and stable in water and oxidative conditions. In click reactions, azide moieties are orthogonal to common functional groups and vigorously reactive with others. Azides are virtually absent from any natural species with bio-orthogonality in vitro and vivo applications.
CuAAC reactions involve the formation of 1,2,3-triazole of a rigid five-membered heterocycle under mild conditions. For functionality, the azides are easy to introduce, stable in water and oxidative conditions, and vigorously reactive. Such triazoles are isosteres of peptide bond with similar planarity of the amide moiety, but less prone to hydrolytic cleavage.
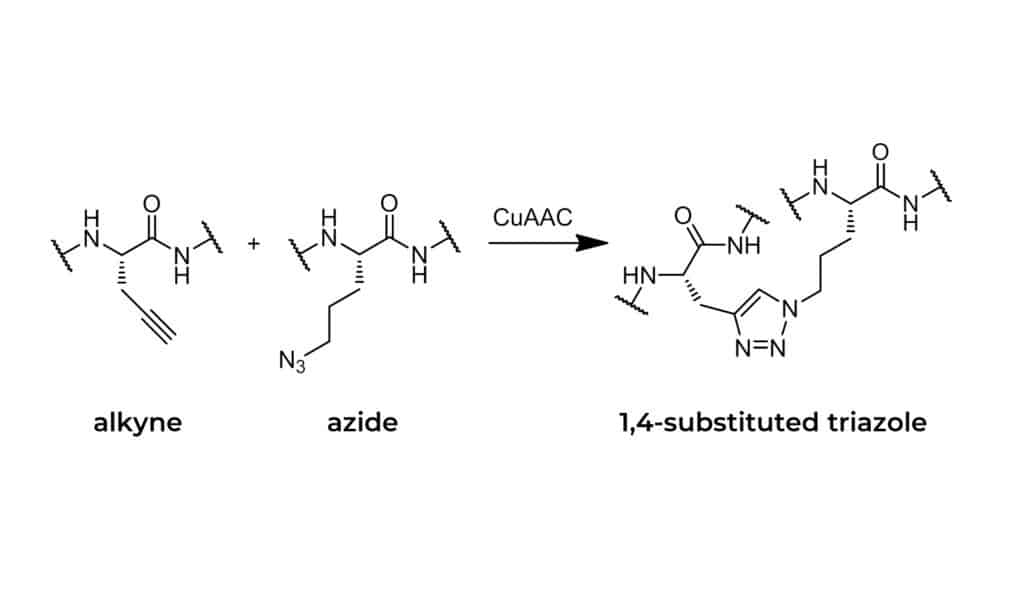
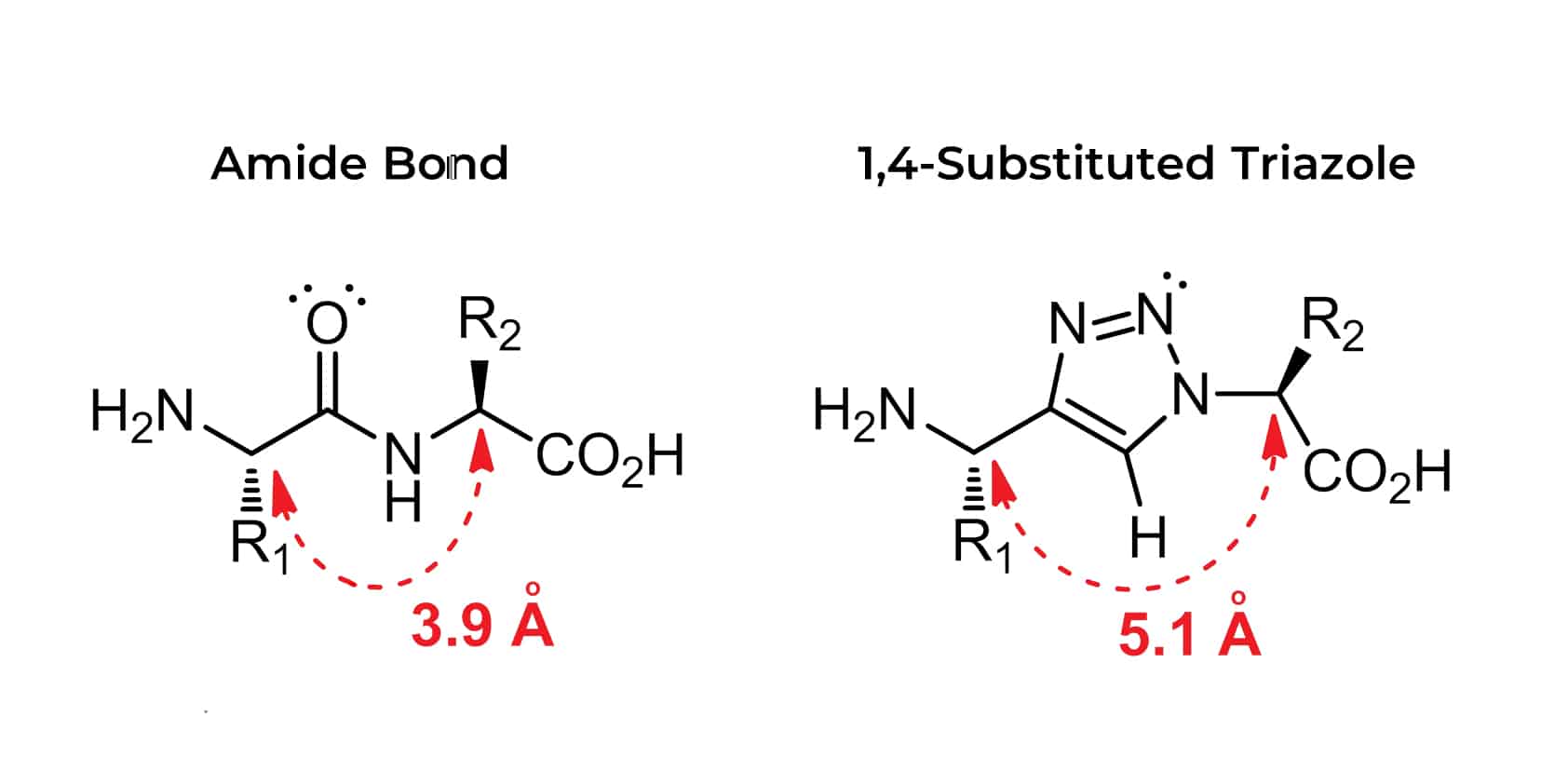
Because the triazole bond has characteristics of high robustness, resemblance to amide bond, and potential biological properties. This makes triazole linkage not only a benign and easily synthesis linker, but also an integral part of click chemistry. Comparing the relative planarity, strong dipole moment (~5D) and hydrogen bonding ability, the 1,2,3 -triazole has a physicochemical resemblance to the amide bond.
Copper Free Click Chemistry
Copper free click chemistry is an alternative technology to click chemistry, this method has a lower activation barrier and free cytotoxic transition of metal catalyst. Copper-free click chemistry is based on the reaction between cyclooctyne and phenyl azide, with the final product of 1-phenyl-4,5,6,7,8,9-hexahydro-1H-cycloocta[d][1,2,3]triazole.
CuAAC click reactions have limitation in vivo applications widespread, because of the cytotoxicity of copper. The presence of copper or reducing agents result in degradation or aggregation of the targeting bio-molecules. Therefore, copper free click chemistry will overcome these challenges. Copper-free click chemistry applies the reaction of cyclooctynes (DIBAC or MOFO) with azides at ambient temperature, this technology never requires Cu catalyst.
Copper Free Click Chemistry Linker
As Copper-free Click Chemistry is the reaction of a cyclooctyne moiety with an azide-labeled reaction partner, there are five main copper free click chemistry linkers:
BCN Linker
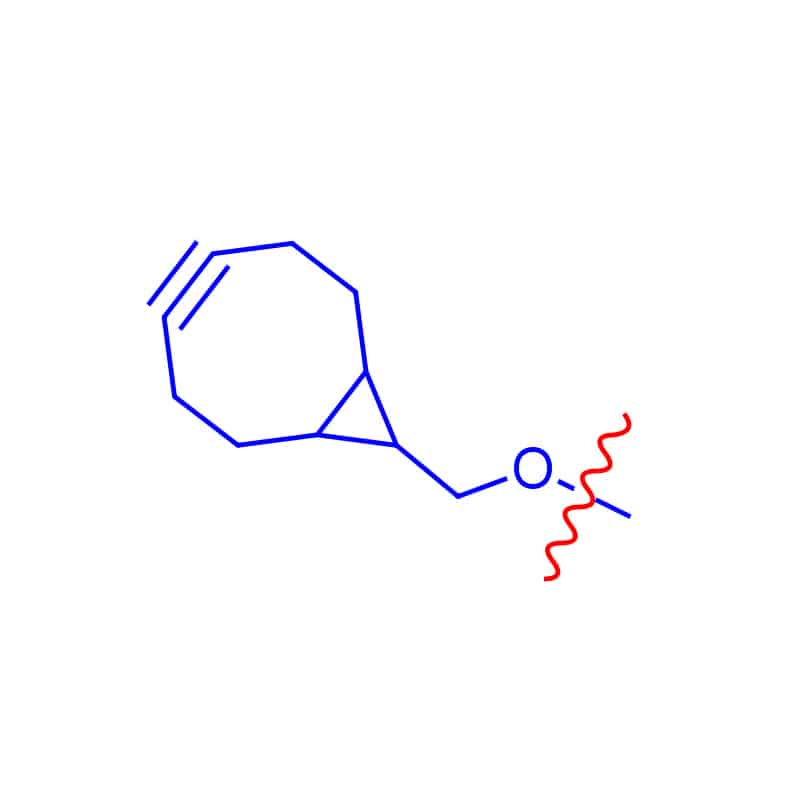
The BCN group can react with azide-tagged biomolecules without copper catalysts. BCN linkers can connect with various functional groups like amine, aminooxy, acid, NHS ester.
Cyclopropene Linker
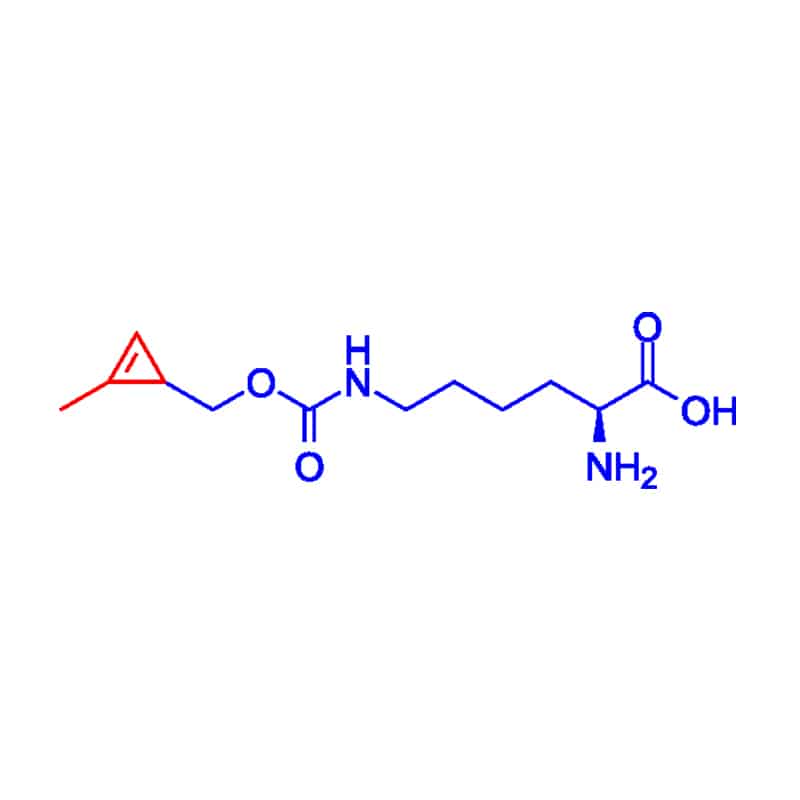
Cyclopropene is the smallest unsaturated carbocyclic compound, it has high reaction in additions, cycloadditions, and ring opening reactions.
DBCO Linker
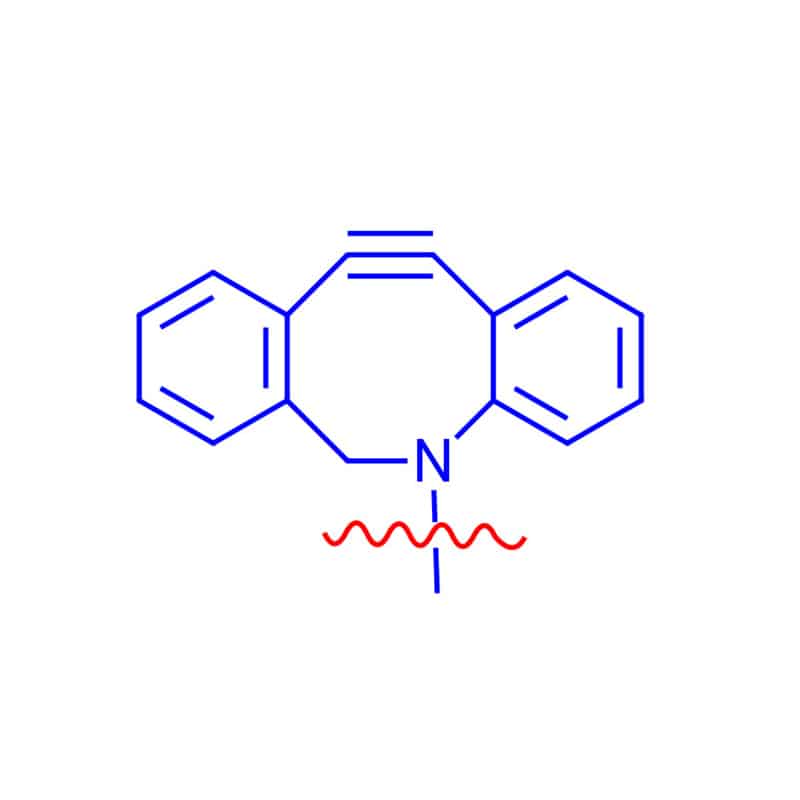
DBCO linkers can react with azide-modified biomolecules without toxic Cu catalysts, DBCO reagents are ideal for living cells and organisms.
TCO Linker
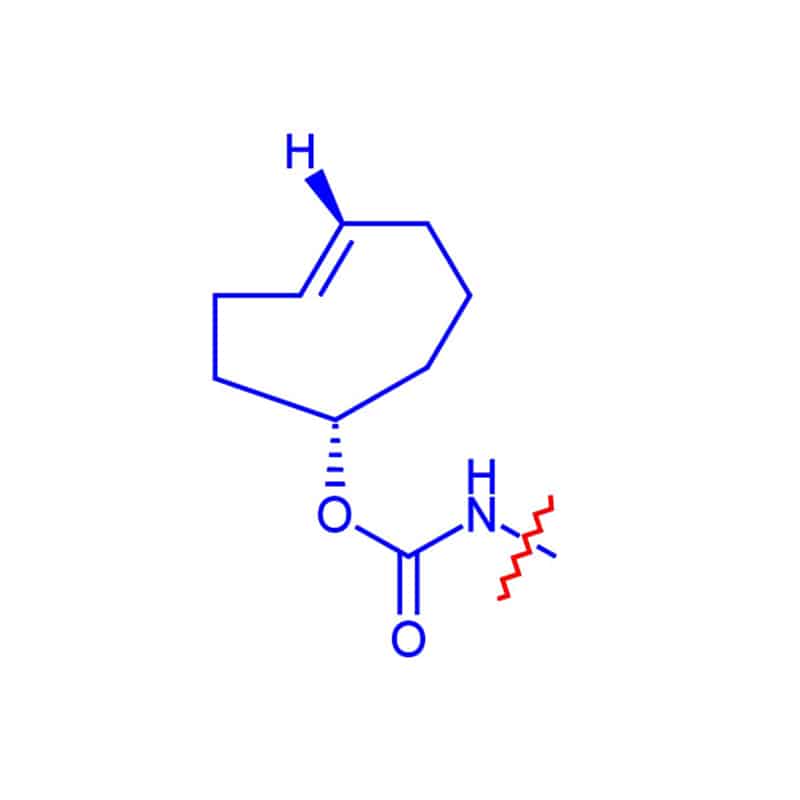
TCO linkers are bioorthogonal agents for tetrazine. The cycloaddition reaction between tetrazine and trans-cyclooctene (TCO) is applied widely for bioconjugation, molecular imaging and cell-based diagnostics.
Tetrazine Linkers
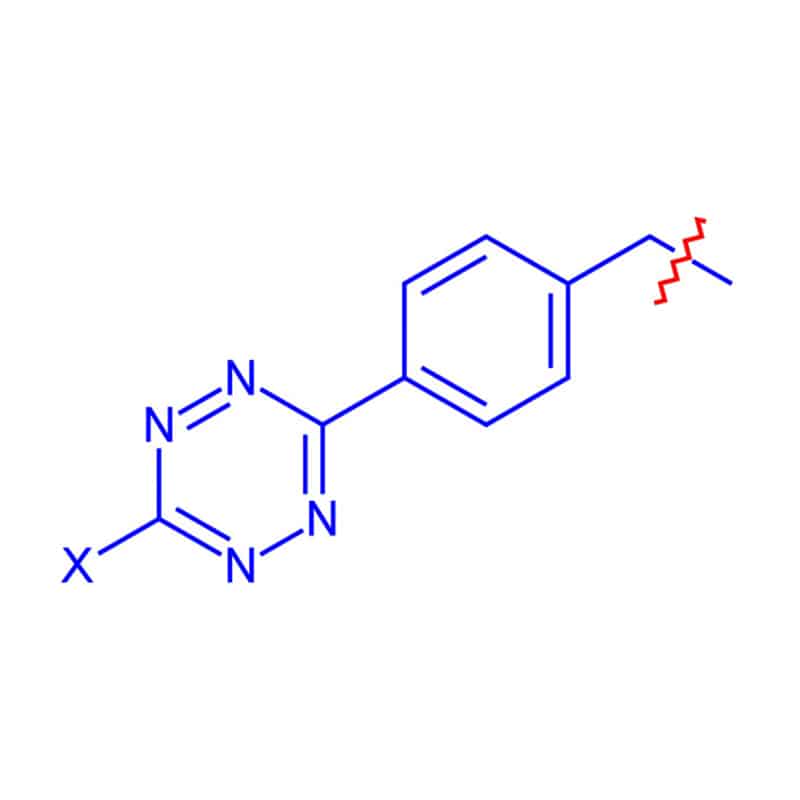
Tetrazine linkers are the bio-orthogonal ligation tools for TCO and Cyclopropene. Tetrazine linkers are chemo-selective, and react specifically with trans-cyclooctene (TCO). These are ideal tools for research in bioconjugation, cell engineering and site/target specific conjugations.
Peptide Click Chemistry
Click chemistry explore various approaches for peptide and protein modifications, this technology can combine with other techniques to create multi-component system with complex structures and unique functions. The common peptide synthesis in click chemistry, including:
- Peptides are converted to azido derivatives, then click with appropriate substrate with alkynyl group, or vice versa.
- Apply intermolecular and intramolecular click reactions in azide or alkyne containing amino acids to build blocks during peptide synthesis.
- Building blocks by clickable moieties for construction of sidechain modified peptides, interside-chain peptide chimera, peptide small molecule conjugates, cyclic peptides.
- Click reaction with solid phase resin modified peptides for clickable/modified peptides.
The critical applications of click chemistry in peptides synthesis including: chemical ligation, cyclization, and bio-conjugation. Others are isotope labeling conjugation for imaging, peptidomimetics synthesis on triazole backbone, conformation and backbone modification. There are various reagents and building blocks in peptide click chemistry:
- ω-azido-α-amino acids
- PEG and spacer azides and alkynes
- azide- or alkyne-modified fluorescent dyes and quenchers, nucleosides and nucleotides
- alkyne or azide-containing chemical modification reagents
- diazo transfer reagents
- propargyl derivatives of amino acids (O-propargylserine, glutamic acid bispropargyl amide)
Chemical Ligation and Peptide Modification
Ligation is linking two or more peptide fragments to form a larger peptide chain. Click chemistry is convenient for peptide linkage synthesis. The peptide fragment with an alkyne group can ligate to another peptide with the N-terminal azide moiety, and result in a triazole linker with two peptide units.
In addition, multimeric peptides are synthesized by incorporating orthogonal sidechain protecting groups, then de-protection selectively, and click reaction of alkyne functional attachment with N-terminal azide peptides. There are typical examples of peptide ligation as following:
- Clickable RGD peptides (Lys side chain reaction with azido acetic acid) link to another peptide fragment.
- Cell-permeable peptides by clicking reactions between alkynyl-modified peptide drug (propargylamine or 1-(2-nitrophenyl)propargyl alcohol) and nona-arginne with azide group modification.
- Neurotensin (8-13)-containing heterodimers from click reaction of alkyne-neurotensin and azide of Plk1-PBR binding phosphorylated hexapeptide.
- Modification of peptide PEGylation, apply azido-PEG to cleave PEGylated peptide from solid-phase synthesis resin.
Click peptide chemistry has tremendous potential for various chemical modifications of peptides and proteins. Including: attaching ligands, liphophilic/hydrophilic groups or linkers.
Peptide Cyclization
Peptide cyclization can increase the clinical efficacy and bio-availability. Click reactions are exploited in different macro-canalization, such as:
- On-resin cyclization of disulfide-containing peptide
- Novel heterodetic cyclopeptides with a triazole bridge
- Vancomycininspired mimics generation by tripetide cyclization
- On-resin cyclization of ascular endothelial growth factor (VEGF) receptor-1 etc
In addition, macrocyclic heterodimers in click-mediated macrocyclization reactions also open up new prospects of complex peptide synthesis.
Bio-Conjugation
Bioconjugation is the process of synthetic molecules to attach to biological targets, or biomolecules to link together. Click chemistry on peptide bioconjugation has extensive applications:
- Arginine-rich TAT peptides conjugation with oligonucleotides, cytotoxic drugs, kinase inhibitors for cell penetration therapeutic
- Chemical methods for mimics of glycopeptides and glycoproteins with homogeneous structure
- Synthesis of complex cyclopeptide-centered multivalent glycoclusters
- Self-assembling peptide fibers incorporate multiple clickable peptides non-covalently and stoichiometrically without structure and stability disruption.
- Conjugations between fluorescent molecules and peptides in mild conditions.
QYAOBIO has thorough expertise in peptide synthesis for superior peptide antigens for antibody generation. We have leading expertise in design and synthesis of complex peptide antigens.
Peptidomimetics Design & Synthesis
As relative planarity and large dipole moment, the 1, 2, 3-triazole has physicochemical resemblance to an amide bond. Consequently, the triazole linkage has particular applications in peptidomimetics.
- The triazole unit has resistance to enzymatic degradation, hydrolysis, and oxidation, this makes it an attractive moiety to replace labile linkers in biologically active compounds.
- Conjugation strategy in design and synthesis of complex biomimetic architectures, triazole linkage replace peptides and phosphodiester bonds.
- Unique conformational characteristics of trizlole units in click reaction: helical components, β-turn units, cis/trans-prolyl ratio modifiers.
Modified peptides with triazole rings on backbones or side-chains are excellent candidates for new antimicrobial agents, such as triazole units are effective alternatives for peptide portions in HIV-1 protease inhibitors.
Peptide Radio-labeling and Imaging
The CuAAA is ideal for ligation reaction in radiolabeling sensitive biomolecules. Alkyne or azide derivatives with radio-isotope-containing are applied to label biomolecules like folic acid, peptides, proteins, and glycopetides. The 11C isotope label can be introduced by converting [11C]-CH3 into [11C]-CH3 N3 by nucleophilic substitution, then reacting with an alkyne-modified peptides. 18F labeling for PET imaging is attained by azidomethyl-4-[18F]-fluorobenzene react with modified peptides. However, Chelators as DOTA will form complex with the Cu catalyst, these compounds are difficult to achieve in click reactions.
Call Us
+86(021)-50795728
+86(027)-60707970
