FRET Peptide
FRET peptides create a new strategy in enzyme studies.
FRET peptides (Quenched fluorescent peptides) are the suitable substrates in enzyme studies. FRET peptide synthesis requires the peptides are conjugated with both a fluorophore and a quencher dye. Moreover, the fluorescence/quencher pars must have distinct overlap between the emission spectrum of flurophores and the absorbance spectrum of quenchers.
Fluorescence Resonance Energy Transfer (FRET)
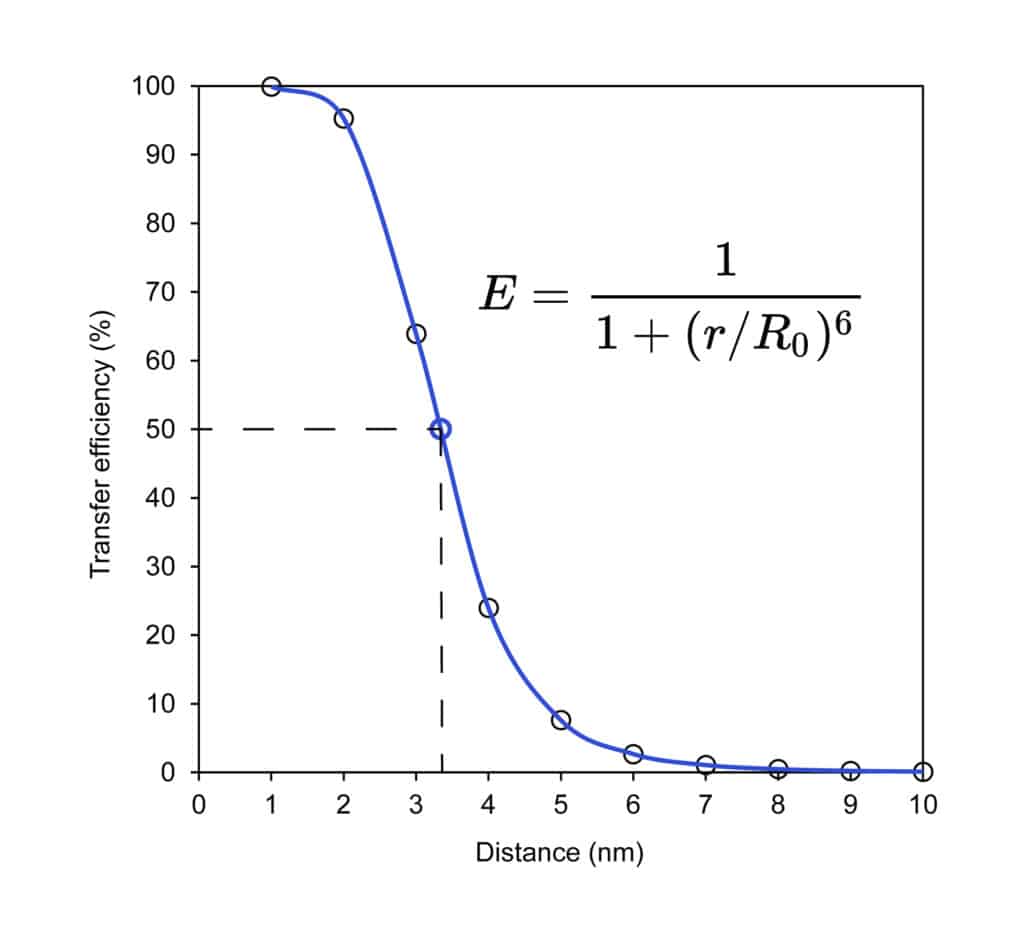
FRET (Fluorescence Resonance Energy Transfer) is a distance-dependent dipole-dipole interaction, this interaction results in energy transfer from initial excited donor molecules to acceptor molecules without photon emission. This method can detect the molecular interactions in the nano-meter range. Fluorescence Resonance Energy Transfer is a detection method of the ‘distance-dependent’ interaction between the two distinct dye molecules in excited states: the fluorophore and the quencher.
Therefore, once the fluorophore and quencher are conjugated to the same peptide with limited distance, the quencher can block the emission efficiently from the fluorophore. However, once the peptide bond is cleaved by enzymatic degradation, the distance between fluorophore and quencher will increase significantly, then the fluorophore is activated. In this case, the fluorescence signal can be detected continuously for quantification of the enzyme activity.
QYAOBIO provides FRET peptide synthesis with a wide variety of fluorophore/quencher pairs
FRET Principle
FRET occurs once the donor (fluorophore) and the acceptor (quencher) are in proximity within 10-100Å. In this case, the energy from the excited fluorophore will transfer to the quencher without fluorescence emission.
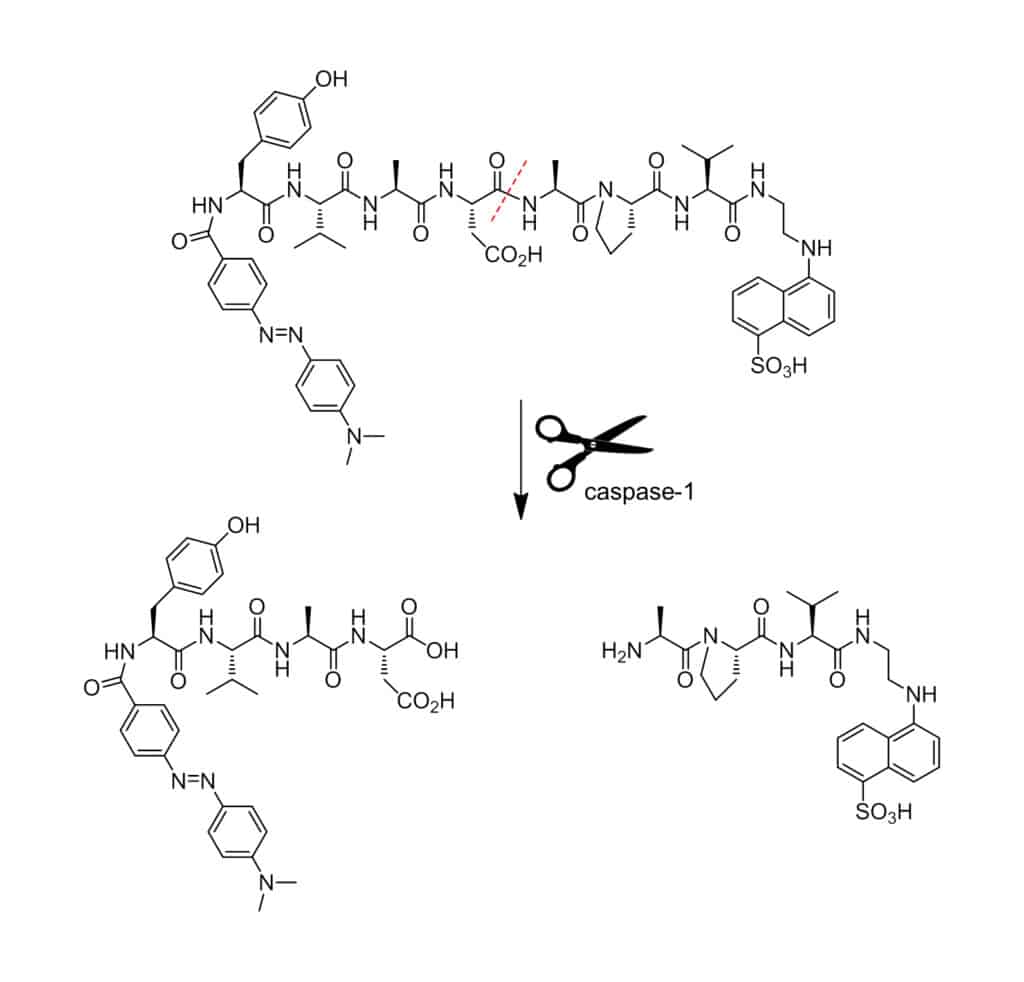
Once the donor and acceptor are on the same peptides as a protease substrate, enzymatic hydrolysis will result in fluorescence emission because of spatial separation of donor and acceptor.
FRET Structural Characteristics
Normally, FRET peptide is labeled with both a donor molecule and an acceptor/quencher molecule. The donor and acceptor pairs are two difference dyes types,
- If the accoptor is a non-fluorescent dye (quencher), the transfer energy is converted into molecular vibrations. Therefore, once the FRET is terminated by separation of donor and acceptor, there is an increased detection of donor fluorescence.
- When both the donor and acceptor dyess are fluorescent, the transfer energy is emitted as light with longer wavelength. Consequently, the alternation of intensity ration can be detected in donor and acceptor fluorescence.
In order to ensure the efficient FRET quenching, the fluorophore and quencher molecules must be close at approximately 10-100 Å.(Å: angstrom). Besides, the absorption spectrum of the quencher must overlap the emission spectrum of the fluorophore. Therefore, in the donor-quencher FRET designing system, it is critical to compare the donor’s fluorescence spectrum with the quencher’s absorption spectrum.
The standard dye combinations of FRET:
- Fluorophore & Dabcyl or Dabsyl b
- Fluorophore & Tamra c
- Methoxy-counmarin-acetic-acid (MCA) & 2,4-Dinitrophenyl (DNP)
FRET Action Mode
In FRET peptides, the fluorescent donor group attached to the peptide transfers energy to the quenching acceptor in sequence with resonance mechanism. This process happens once the emission spectrum of the fluorophore overlaps with the absorption spectrum of the acceptor. The FRET peptides exhibit internal fluorescence quenching in intact formation. But cleavage of any peptide bond between the donor and acceptor pairs will liberate fluorescence with continuous detection, this allows the quantitative measurement of enzyme activity.
FRET Peptide
QYAOBIO has extensive knowledge and experience in the design and synthesis of FRET peptides. We provide a wide range of FRET substrates to satisfy your research requirements, as custom FRET peptides and custom FRET sequences. We provide a free consultation to assist your design of FRET peptides and selection of FRET pair.
Benefits of FRET Peptides
FRET and fluorogenic substrates have higher sensitive than chromogenic substrates with linear dynamic range and great reproducibility. FRET peptides are applied in various applications with high benefit, especially as protease substrates in high-throughput inhibitor screening & drug discovery. The main benefits of FRET peptides are:
- Unique quenchers tandem with popular dyes
- High quality HiLyte™ Fluor dyes
- Affordable price of Cy dyes with identical structure
- Longer wavelengths for higher sensitivities
- Easy adaption to high throughput screening assays
Applications of FRET Peptides
FRET peptides are suitable substrates in enzyme studies:
- Detection and measurement of protease activity.
- Target screening and discovery of protease-based drug.
- High throughput screening of protease inhibitors.
- Functional and kinetic characterization of peptidases, proteases, kinases, phosphatases.
- Screening and detection of new proteolytic enzymes.
- Conformational investigation of peptide folding.
FRET Substrates
We provide a full-rage of dye-quencher FRET pairs and fluorogenic substrates. The common FRET substrates including:
| Name | Abs/Em | Applications |
| ABZ | 320/420 nm | FRET |
| Me-Abz | 340 -360/440 -450 nm | FRET |
| MCA | 325/ 392 nm | FRET |
| EDANS | 340/490 nm | FRET |
| FITC | 490/520nm | FRET |
| Dansyl | 342/562 nm | FRET |
| DMACA | 350/465 nm | FRET |
| TRP | 280/360 nm | FRET |
| Lucifer Yellow | 430/520 nm | FRET |
ABZ
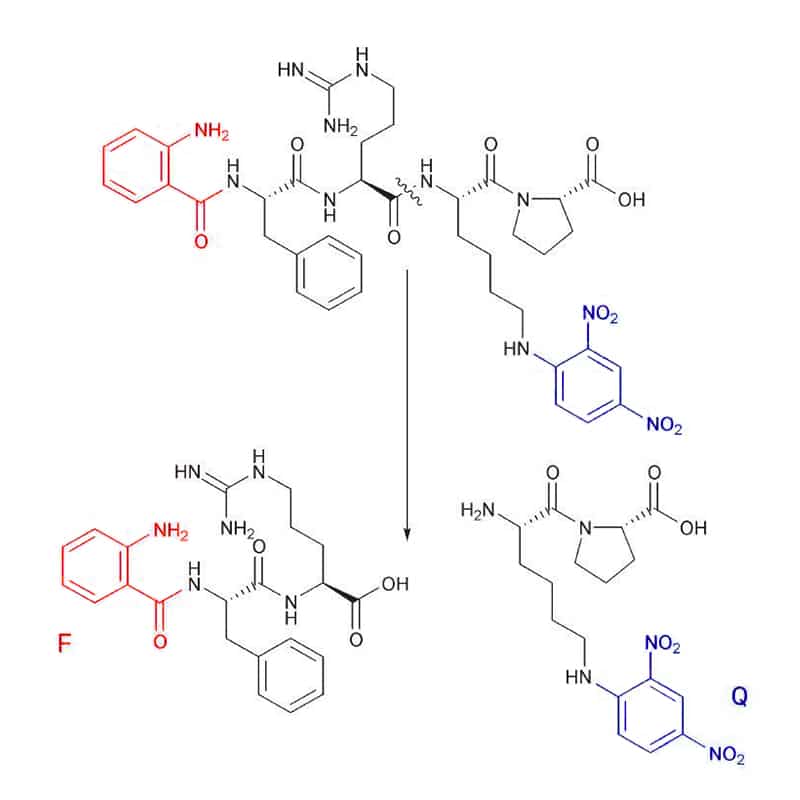
Abz (F) substrates can combine with a number of quenchers (Q), like Dnp (2,4-dinitrophenyl), EDDnp (N-(2,4 dinitrophenyl)ethylenediamine), 4-nitro-phenylalanine, or 3-nitro-tyrosine. Substrate cleavage is detected at 420 nm, with the excitation wavelength of 320 nm.
Me-Abz
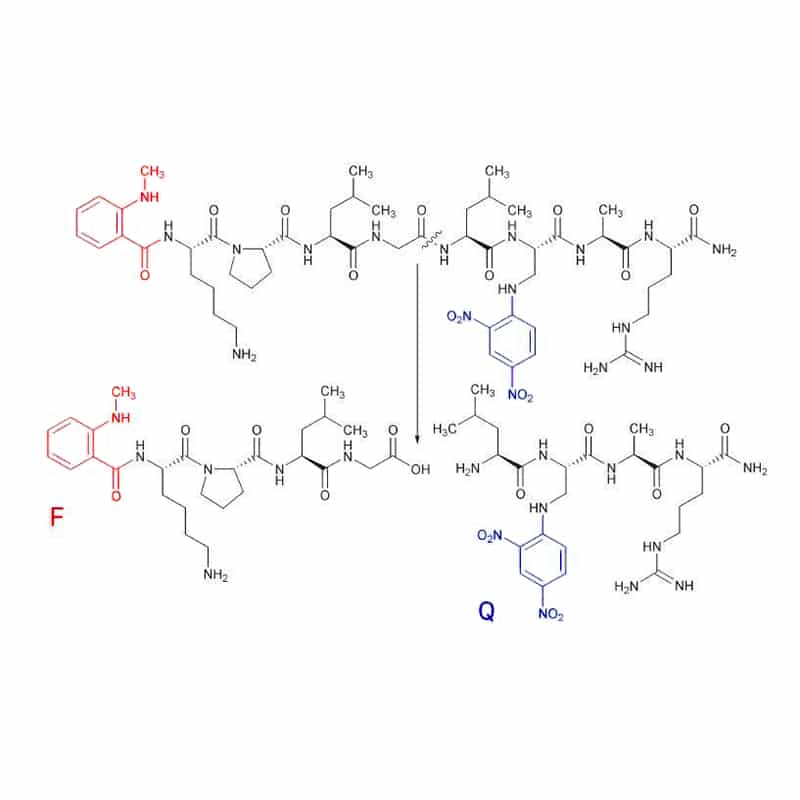
N-Me-Abz substrates generally combine with Dnp as quencher (Q). This fluorescent group (F) can link to either the N-terminal amino group or the ε-amino group of a lysine residue. Substrate cleavage can be detected at 440 -450 nm, with the excitation wavelength of 340-360 nm.
MCA
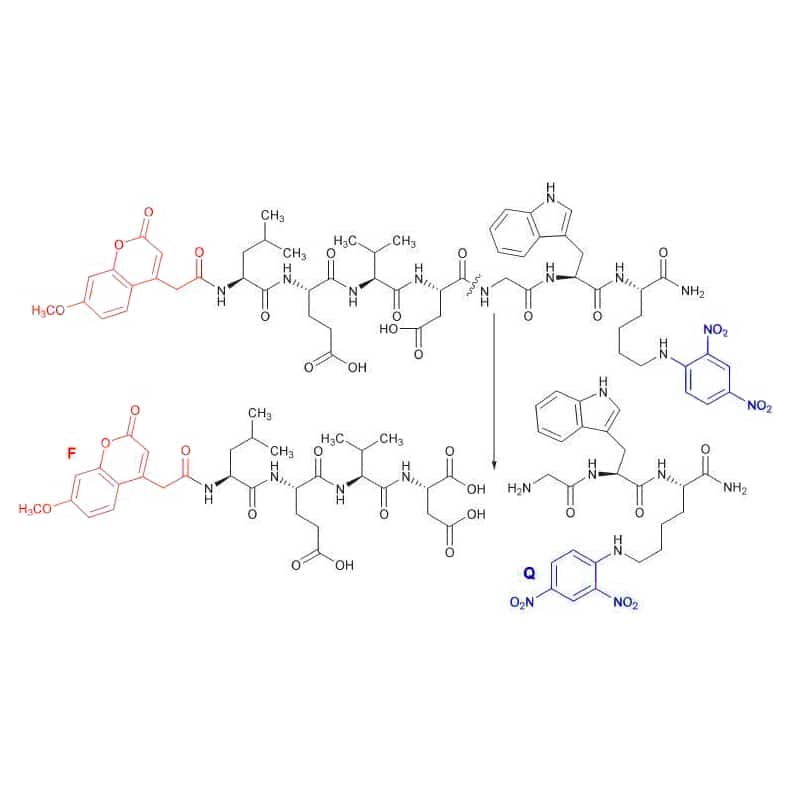
MCA (F) is usually bound to the N-terminal amino group of peptide, and quenched by DNP (Q). The cleaved peptides with MCA group is assayed at 392 nm using excitation wavelength of 325 nm.
EDANS
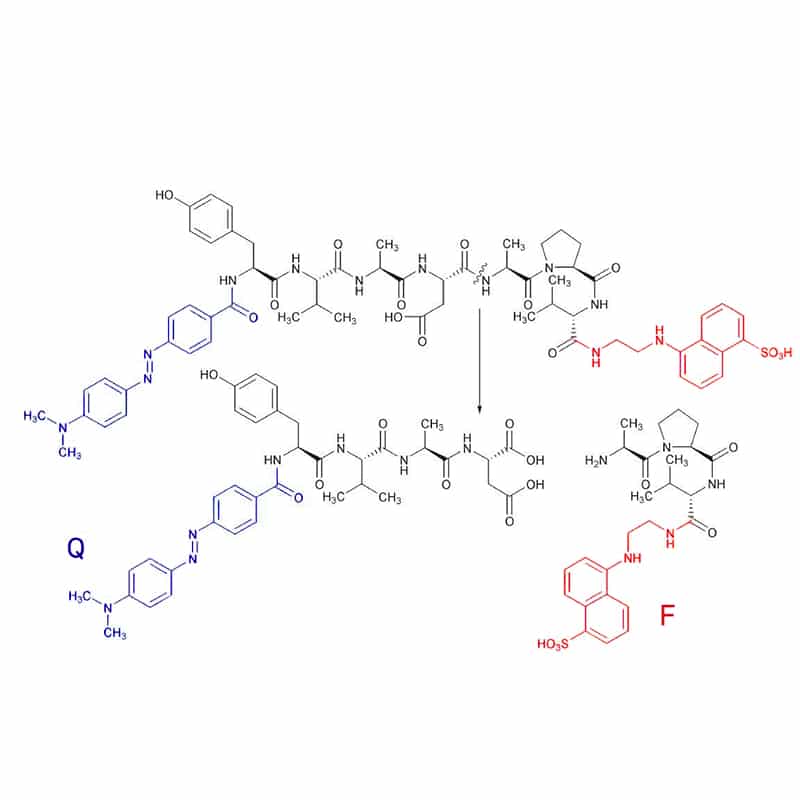
The EDANS group is generally quenched by the DABCYL (4-(4 dimethylaminophenylazo) benzoyl) group (Q). The DABCYL group is attached to the N-terminus, while the EDANS group conjugated to the C-terminus of peptide substrates. Substrate cleavage is detected at 490 nm with excitation of 340 nm.
FITC
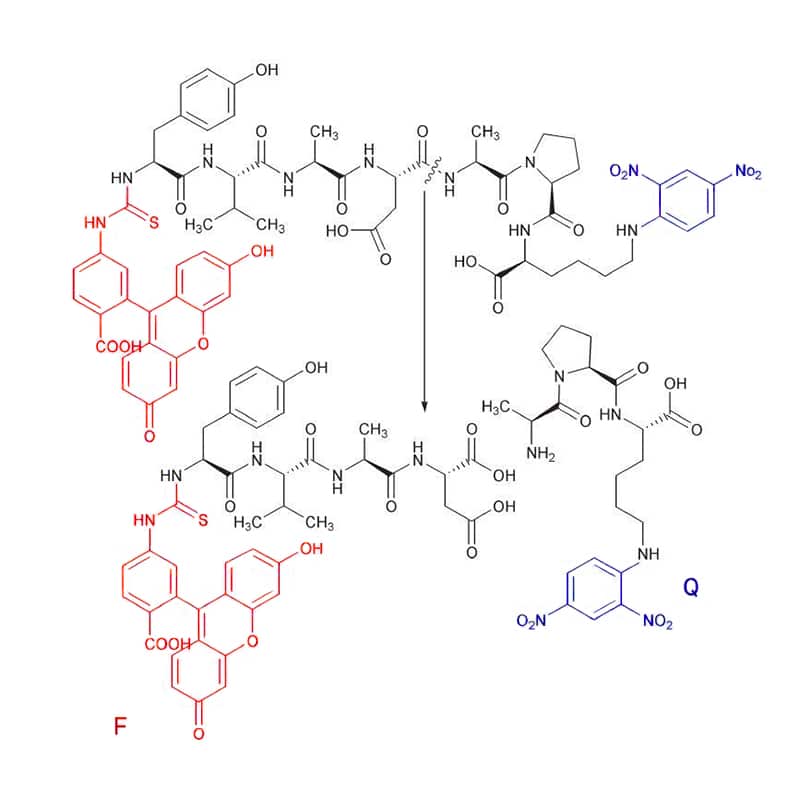
There are only few FITC substrates, the FITC (F) is quenched with Dnp (Q). Substrate cleavage can be detected at 520 nm with excitation wavelength of 490 nm.
Dansyl
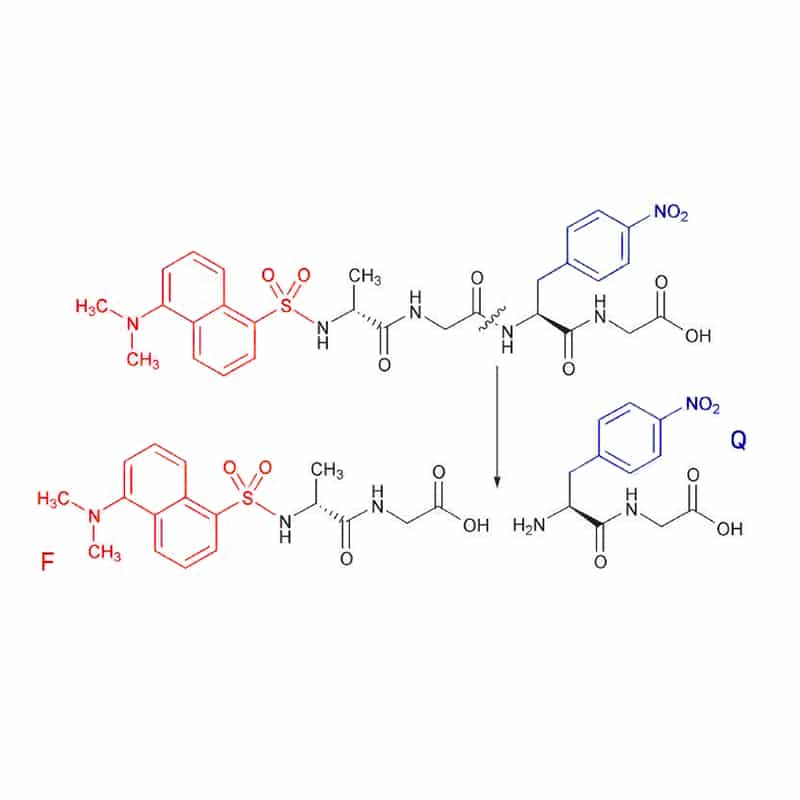
In the rare case, the fluorescent dansyl group (F) acts as the donor with 4-nitro-phenylalanine as acceptor. The substrate cleavage is assayed at 562 nm, with excitation at 342 nm. However, the Dansyl is applied commonly as a quencher for tryptophan fluorescence.
DMACA
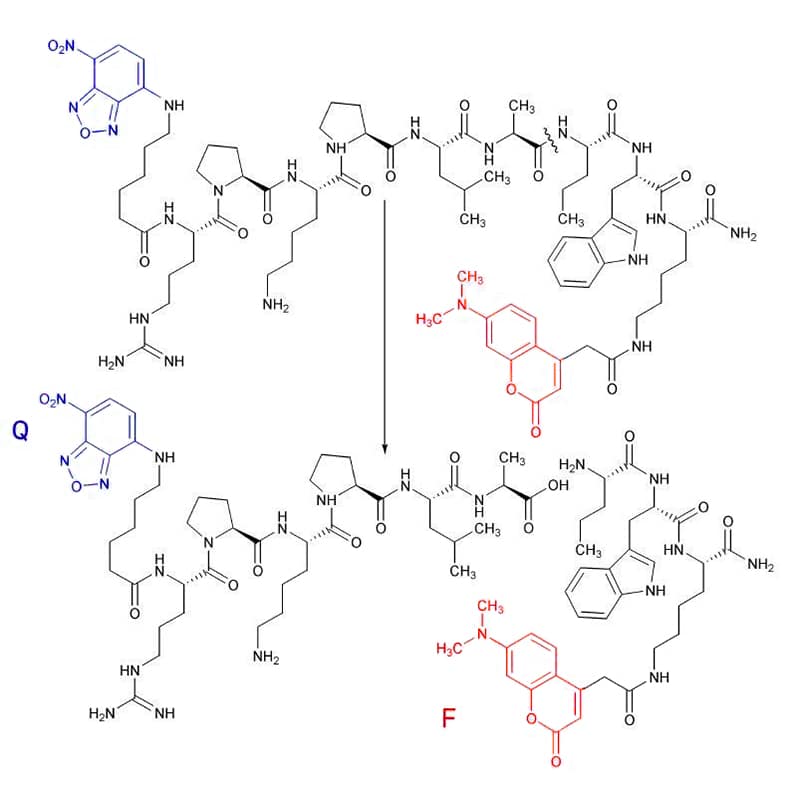
DMACA is quenched by NBD (7-Nitro-benzo[2,1,3]oxadiazol-4-yl) (Q), it is detected fluorometrically at 465 nm with excitation at 350 nm.
TRP
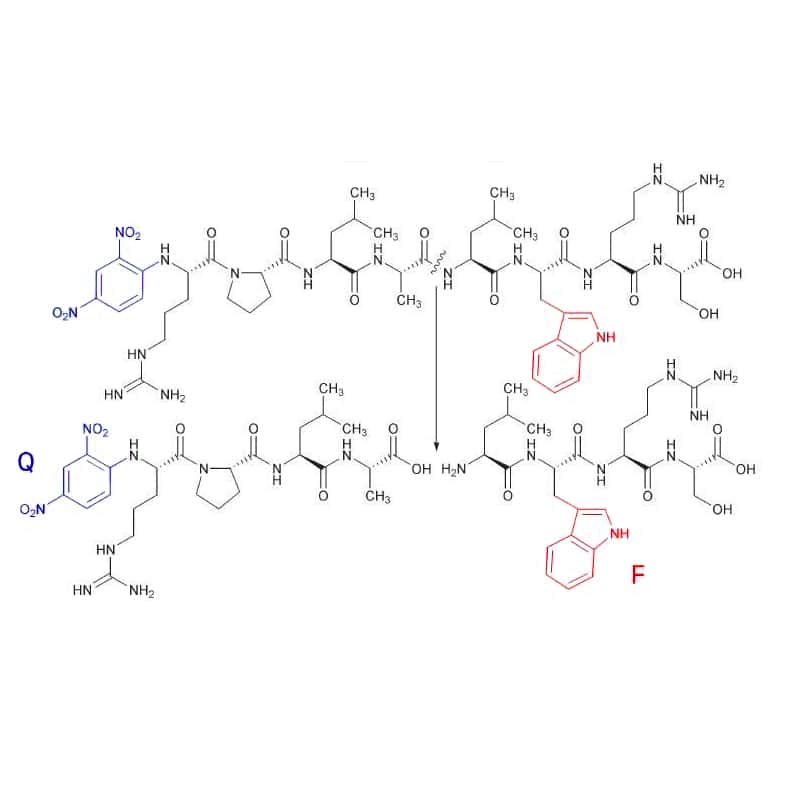
Tryptophan (F) is a fluorescent amino acid with DNP as a quencher (Q), it hascleavage detection at 360 nm with excitation of 280 nm.
Lucifer Yellow
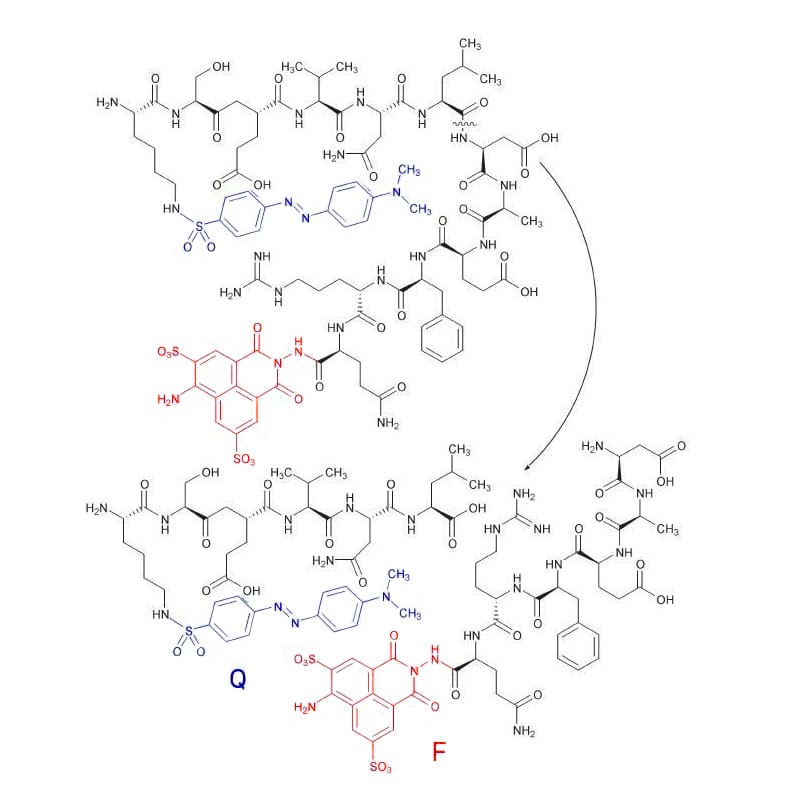
Lucifer Yellow (F) is quenched efficiently with Dabsyl (Q), it is detected at 520 nm with excitation at 430 nm.
TR-FRET Peptides
TR-FRET is the combination of Time Resolved Fluorescence (TRE) and Fluorescent Resource Energy Transfer (FRET) technologies. Time-resolved FRET (TR-FRET) utilizes the long-lived fluorophores of lanthanide elements to delay measurements by 50-150 µs. Therefore, TR-FRET peptides are labeled with a well-defined fluorescent donor of lanthanide metals, which delays the measurements by this timeframe. This time delay results in the cleared singal of most nonspecific short-lived emissions. TR-FRET eliminates background fluorescence with better data quality.
In TR-FRET, lanthanide metal like Europium (Eu) is applied to replace the fluorescent dye as the donor molecule. This Europium florescence is longer-lived than the conventional fluorophore. As the large stokes shift of Europium (Eu), TR-FRET provides a high signal-to-noise ratio with minimal crosstalk between excitation and emission wavelengths. TR-FRET gives rise to flexible, reliable and sensitive assay with fewer false results in high-throughput applications.
DOTA-Peptide for TR
DOTA is a metal chelator with binding to lanthanide metals, we can synthesized any DOTA-peptide for any TR-FRET applications.
TR-FRET Application
- Macro-molecule interactions between protein-peptide or protein-protein: each interacting partner couples with one of the TR-FRET pairs with measurement of transfer energy.
- Protease activity measurement: TR-FRET is applied to generate peptides with specific substrates of know proteases.
- Protease-based drug target screening and discovery.
TR-FRET Sensitivity
In TR-FRET, the donor molecule is Lanthanide metal like Europium. The resonance energy transfer from the donor to the acceptor of the Quencher or fluorescent dye, then the acceptor emits light in turn at the corresponding emission wavelength.
Biological samples like fluid, serum, cells, and tissues will generate background fluorescence during the conventional FRET assay, this results in low signal-to-noise ratio. However, in TR-FRET assays, the fluorescent signal of Lanthanide metals is long-lived, and it can be detected once background fluorescence of biological samples have decayed. This results in low background noise and improve assay sensitivity of TR-FRET.
Call Us
+86(021)-50795728
+86(027)-60707970
